Market Overview and Dynamics
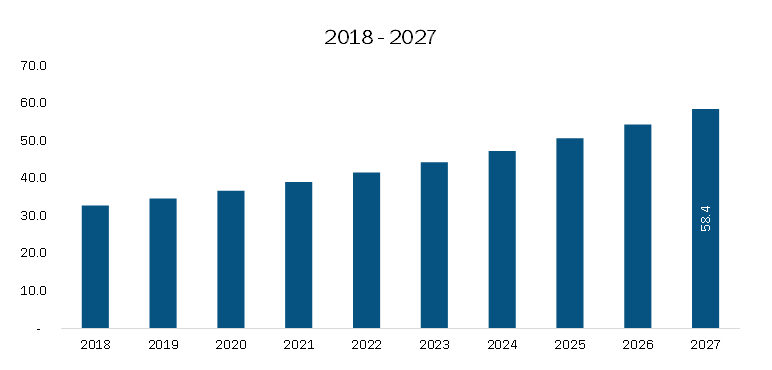
The Europe clinical trial supplies market is projected to grow from US$ 550.28 million in 2019 to US$ 938.54 million by 2027, registering a CAGR of 7.0% during the forecast period from 2020 to 2027. This growth is primarily driven by rising R&D investments by pharmaceutical and biopharmaceutical companies, alongside an increasing number of clinical trials being conducted across the region. However, the market also faced certain setbacks due to the COVID-19 pandemic, which temporarily disrupted clinical trial operations and supply chains, posing challenges to market expansion.
📚Download Full PDF Sample Copy of Market Report @
https://www.businessmarketinsights.com/sample/TIPRE00022074
Effective clinical trial supply management plays a crucial role in minimizing overproduction, avoiding oversupply, and reducing the risk of inventory expiration—an increasingly important consideration given the high costs of drug development. In recent years, a notable trend in the industry has been the growing reliance on outsourcing clinical trial supply operations. Pharmaceutical companies are increasingly partnering with contract research organizations (CROs) to reduce costs, accelerate timelines, gain access to specialized expertise, and improve overall agility.
This outsourcing trend is further supported by collaborative research models, wherein in-house R&D teams work closely with external contract developers. As pharmaceutical and biotech firms continue to increase their R&D expenditures, this model is expected to gain further traction, contributing to market growth. The rise in drug development activities and the expansion of clinical trial programs across Europe are also expected to provide strong momentum to the clinical trial supplies market.
The COVID-19 pandemic initially led to production slowdowns, drug shortages, and a reallocation of resources toward treatments and vaccines for the virus. Many companies were forced to postpone or halt ongoing trials due to disrupted logistics and supply chains. However, with a gradual resumption of trial activities and recovery efforts in the pharmaceutical sector, the market has begun to stabilize. While the pandemic posed short-term challenges, it also underscored the importance of resilient and adaptive supply chain strategies, which may benefit the market in the long term.
|
Segment |
Sub-Segments |
Key Insights (2019) |
|
By Product & Service |
– Manufacturing |
Logistics & Distribution held the largest share and expected to grow at the highest CAGR |
|
By Clinical Trial Phase |
– Phase I |
Phase III dominated and projected to grow fastest due to rising late-stage trials |
|
By Drug Type |
– Small-Molecule Drugs |
Small-molecule drugs led in 2019; biologic drugs expected to grow at highest CAGR |
|
By Application |
– Oncology |
Oncology was the largest and fastest-growing segment, driven by increased cancer trials |
Market Introduction
The clinical trial is an investigation study that defines whether a medical approach, therapy, or device is effective, safe, and useful for human applications. These studies help to find which therapeutic approaches experiment is best for certain diseases. Clinical trial supplies management is necessary for evading overproduction, oversupply, and inventory expiration. With the increasing costs of drug discovery, clinical trial supplies are obtaining more importance.
Europe Clinical Trials Supplies Strategic Insights
Strategic insights for the Europe Clinical Trials Supplies provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
Regional Insights for Europe’s Clinical Trial Supplies Market
Defining the geographic scope of Europe’s clinical trial supplies market means pinpointing the precise territories where a company operates and competes. Success hinges on recognizing local nuances—ranging from preferred plug types and backup-power expectations to economic conditions and regulatory frameworks—and tailoring strategies accordingly. By identifying underserved regions or tailoring offerings to meet specific local needs, businesses can allocate resources more efficiently, run highly targeted marketing campaigns, and strengthen their position against regional competitors, ultimately accelerating growth in those focus areas.
About Us:
Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Défense; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications
Author’s Bio
Akshay
Senior Market Research Expert at Business Market Insights