Market Overview and Dynamics
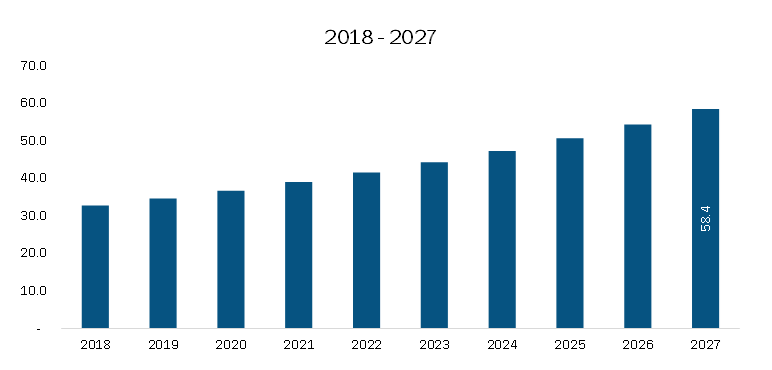
The Europe clinical trial supplies market is projected to grow from US$ 550.28 million in 2019 to US$ 938.54 million by 2027, registering a CAGR of 7.0% from 2020 to 2027. This growth is primarily driven by rising R&D investments by pharmaceutical and biopharmaceutical companies and an increasing number of clinical trials across the region. However, the COVID-19 pandemic has presented challenges that temporarily restrained market expansion during the forecast period.
📚Download Full PDF Sample Copy of Market Report @
https://www.businessmarketinsights.com/sample/TIPRE00022074
Effective clinical trial supplies management is crucial to avoid overproduction, oversupply, and inventory wastage, especially as the costs of drug discovery continue to rise. In recent years, pharmaceutical companies have increasingly outsourced clinical trial activities to control costs, accelerate development timelines, and gain access to specialized expertise and technology. This shift toward outsourcing has fueled the growth of the clinical trial supplies market, supported by greater R&D spending and a rising volume of clinical trials. Additionally, collaborative research models between sponsors and contract developers have emerged as a notable trend, further boosting market activity.
The COVID-19 pandemic initially disrupted supply chains and forced many pharmaceutical companies to postpone clinical trials, leading to temporary drug shortages and reduced productivity as resources were redirected toward COVID-19 treatments. However, as operations resume and the industry recovers, both the positive and negative impacts of the pandemic are shaping new practices and driving renewed momentum in the European clinical trial supplies market.
Market Introduction
Clinical trials are research studies designed to determine the safety, effectiveness, and overall value of medical treatments, therapies, or devices for human use. These trials are essential for identifying the best therapeutic approaches for specific diseases. Effective clinical trial supplies management is crucial to prevent overproduction, oversupply, and inventory waste due to expiration. As the costs of drug discovery continue to rise, the role of clinical trial supplies has become increasingly significant.
Europe Clinical Trials Supplies Strategic Insights
Strategic insights for the Europe Clinical Trials Supplies provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
Market leaders and key company profiles
· THERMO FISHER SCIENTIFIC INC.
- UDG Healthcare plc
- Catalent Inc.
- pci Pharma Services
- Almac Group
- PAREXEL INTERNATIONAL CORPORATION
- Biocair
- Owens & Minor Inc
- Rubicon Research Pvt. Ltd
- CLINICAL SUPPLIES MANAGEMENT HOLDINGS, INC.
Europe Clinical Trials Supplies Regional Insights
The geographic scope of the Europe Clinical Trials Supplies refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
Key Market Segments
Based on product & service, the Europe clinical trial supplies market is segmented into manufacturing, packaging & labelling, and logistics & distribution. The logistics & distribution segment held the largest share of the market in 2019; the same segment is anticipated to register the highest CAGR in the market during the forecast period.
In terms of stage, the Europe clinical trial supplies market is segmented into phase I, phase II, phase III, and bioequivalence studies. The phase III segment held the largest share of the market in 2019 and is estimated to register the highest CAGR in the market during the forecast period.
Based on drug type, the Europe clinical trial supplies market is segmented into small-molecule drugs and biologic drugs. The small-molecule drugs segment held the largest share of the market in 2019, whereas the biologic drugs segment is estimated to register the highest CAGR in the market during the forecast period.
Based on application, the Europe clinical trial supplies market has been segmented into oncology, cardiovascular diseases, neurological disorders, respiratory disorders, and others. The oncology segment held the largest share of the market in 2019 and is estimated to register the highest CAGR during the forecast period.
Major Sources and Companies Listed
A few of the primary and secondary sources associated with this report on the Europe clinical trial supplies market are the European Federation of Pharmaceuticals Industries Associations (EFPIA), European Medical Association (EMA), UK Clinical Research Collaboration (UKCRC) and European CRO Federation (EUCROF).
Reasons to buy the report
- Determine prospective investment areas based on a detailed trend analysis of Europe clinical trial supplies market over the next years.
- Gain an in-depth understanding of the underlying factors driving demand for different addictions therapeutics segments in the top spending countries and identify the opportunities offered by each of them.
- Strengthen your knowledge of the market in terms of demand drivers, industry trends, and the latest technological developments, among others.
- Identify the major channels driving the Europe clinical trial supplies market, providing a clear picture of future opportunities that will help Analyze, resulting in revenue expansion.
- Channelize resources by focusing on the ongoing programs undertaken by the different countries within the Europe clinical trial supplies market.
Can you see this our reports
Europe Learning Management System Market – https://postyourarticle.com/europe-learning-management-system-market-trends-size-segment-and-growth-by-forecast-to-2030-4/
Europe Dental Market – https://github.com/businessmarketinsights985/business-market-insights/issues/18
North America Medical Laser Systems Market – https://findit.com/uwttwdjttmkjdpa/RightNow/northamerica-medical-laser-systemss/ea672c78-2f49-4851-986c-6f17f7e783af
Europe Railway Cyber Security Market – https://businessmarketins02.blogspot.com/2025/05/europe-railway-cyber-security-market.html
Europe Antibiotics Market – https://sites.google.com/view/businessmarketinsights126/home
About Us:
Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Défense; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications
Author’s Bio
Akshay
Senior Market Research Expert at Business Market Insights