

ANVISA, which stands for Agência Nacional de Vigilância Sanitária, is Brazil’s National Health Surveillance
- Role and Responsibilities: ANVISA’s primary mission is to protect public health by regulating the production, marketing, and use of products and services subject to health regulations, including those related to pharmaceuticals, medical devices, cosmetics, food, tobacco, and health services.
- Structure: ANVISA is an autarchy linked to the Ministry of Health and is part of the Brazilian National Health System (SUS).
- Independence: ANVISA is administratively and financially independent, managed by a 5-member board of directors.
- History: ANVISA was established on January 26, 1999.
Website: The official ANVISA website is https://www.gov.br/anvisa/en
Definition of a Medical Device
ANVISA defines medical devices as: “Health products, such as equipment, apparatus, material, item or system with a medical, dental, or laboratory use or application for prevention, diagnosis, treatment, rehabilitation and that does not use contraception and pharmacological, immunological or metabolic means to perform the main function in humans, but can be assisted in their functions by such means.
Medical Device Classifications and Grouping
Medical devices in Brazil are classified into four classes based on the risk they pose to the human body.
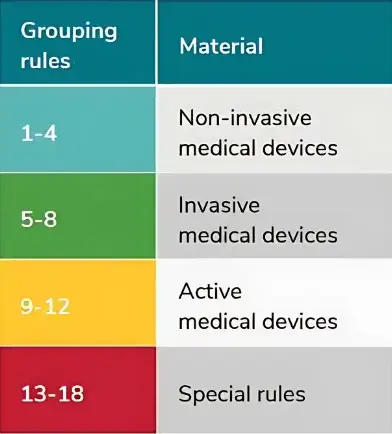
Medical devices also follow the 18 classification rules, which are largely similar to the 18 rules outlined in the European Medical Devices Directive (MDD) 93/42/EEC.
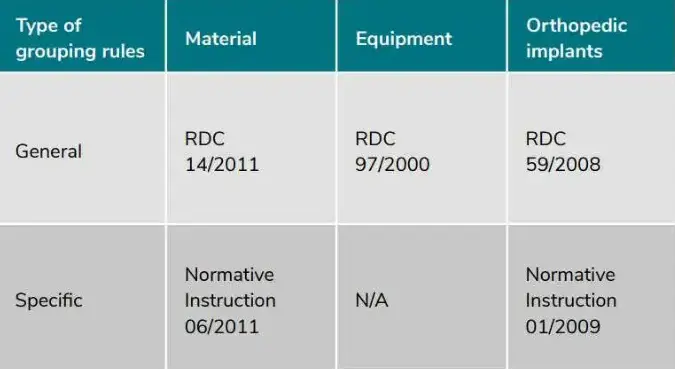
Device registration pathways
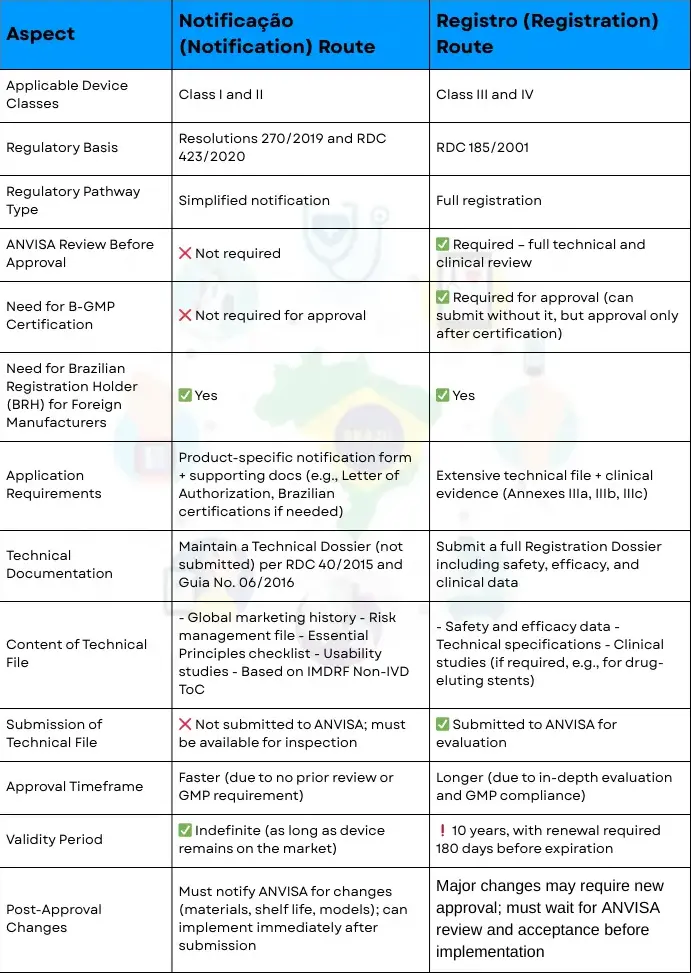
In Brazil, medical devices can be approved through two alternative pathways notificação or registro depending on device classification.

Notificação (Notification) Route
Comparison of Notificação (Notification) vs. Registro (Registration) Routes for Medical Devices in Brazil
Good Manufacturing Practices

Brazil has its own set of Good Manufacturing Practices (B-GMP) requirements, most recently outlined in RDC 665/2022, which replaced RDC 16/2013 as the official Quality Management System (QMS) standard. While Brazil’s GMP framework shares similarities with ISO 13485 and the US FDA’s Quality System Regulation, it is not directly analogous and must be followed independently. According to RDC 183/2017, B-GMP certification is required for all Class III and IV medical devices and applies to relevant manufacturing activities such as design, production, assembly, and labelling. Certification is granted following inspection and is tied to the specific manufacturing facility, production line (e.g., medical device vs. IVD), and product classification, as defined by RDC 39/2013. If any of these element’s change, a new B-GMP certificate is required. Importantly, foreign manufacturers cannot apply directly to ANVISA for B-GMP certification; their Brazilian Registration Holder (BRH) must submit the request. For foreign sites, ANVISA conducts the inspections, whereas for domestic facilities, inspections are carried out by local health authorities (VISA) within the respective Brazilian states. While compliance with GMP is mandatory for all manufacturers, a BGMP certificate is only required for the registration of Class III and IV devices.
INMETRO Certification

INMETRO certification is often required for electromedical devices subject to IEC 60601, as well as some other medical devices. ANVISA requires certain medical devices to be certified by the National Institute of Meteorology Standardisation and Industrial Quality, which is responsible for establishing technical standards in Brazil.
This certification ensures that the devices meet Brazilian standards for safety, quality and performance. The process includes:
Product Testing: Devices must undergo rigorous testing in INMETROaccredited laboratories. Certification Issuance: Once the devices pass the tests, INMETRO issues c certification, which in necessary for both registration and importation of the devices into Brazil.



