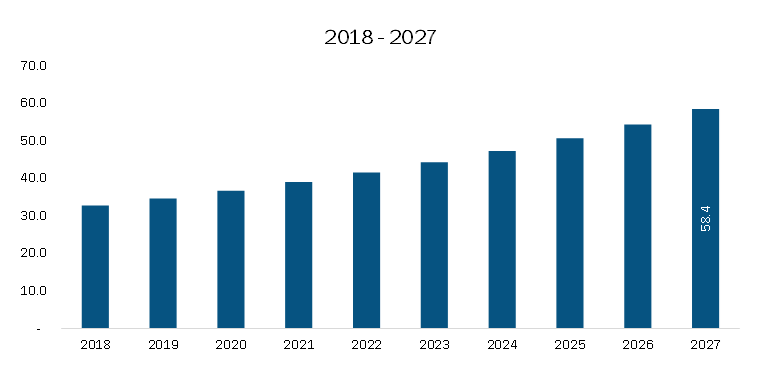
At 8.9% CAGR, the North America Colorectal Cancer Diagnostic Market is projected to be worth US$ 6,621.26 million by 2028, says Business Market Insights
According to Business Market Insights research, the North America colorectal cancer diagnostic market was valued at US$ 4,315.86 million in 2023 and is expected to reach US$ 6,621.26 million by 2028, registering a CAGR of 8.9% from 2023 to 2028. Increasing prevalence of colorectal cancer and launches of new products are the critical factors attributed to the North America colorectal cancer diagnostic market expansion.
📚Download Full PDF Sample Copy of Market Report @-https://www.businessmarketinsights.com/sample/BMIRE00028834
Colorectal cancer is a malignant tumor that develops in colonic or rectal tissues. It is the third most common type of cancer (excluding skin cancer) diagnosed yearly in the US. In 2021, 149,500 adults were diagnosed with colorectal cancer in the country. These numbers include 104,270 new colon cancer cases (52,590 men and 51,680 women) and 45,230 new rectal cancer cases (26,930 men and 18,300 women). During 2012–2016, the incidence of colorectal cancer rose by 2% in adults below 50 and 1% in adults of age 50–64 annually. It is estimated to be the fourth most diagnosed cancer among men and women of age 30–39 in the US. The disease caused 52,980 deaths (28,520 men and 24,460 women) in 2021. Surgery is the most common treatment for all stages of colon cancer. In ideal situations, if the cancer is diagnosed in the early stages, doctors can remove the tumor via surgical procedures. A colonoscopy is an important screening test for colorectal cancer diagnostics, and it has become a part of routine cancer screening. Thus, the rising incidence of colorectal cancer drives the growth of the North America colorectal cancer diagnostics market.
On the contrary, low accuracy of screening based on faecal immunochemical tests (FITs) hurdles the growth of North America colorectal cancer diagnostic market.
Based on modality, the North America colorectal cancer diagnostics market is bifurcated into imaging tests and stool based tests. The imaging tests segment held 94.0% share of North America colorectal cancer diagnostic market in 2023, amassing US$ 4,058.98 million. It is projected to garner US$ 6,248.43 million by 2028 to expand at 9.0% CAGR during 2023–2028. The market for the imaging tests segment is further segmented into colonoscopy, CT colonography, flexible sigmoidoscopy, capsule endoscopy, and others. The market for the stool based tests segment is subsegmented into faecal immunochemical test (fit), guaiac-based faecal occult blood test (gFOBT), and stool DNA test.
Based on end user, the North America colorectal cancer diagnostics market is segmented into hospitals, diagnostic laboratories, cancer research institutes, and others. The hospitals segment held 42.2% share of North America colorectal cancer diagnostic market in 2023, amassing US$ 1,820.01 million. It is projected to garner US$ 2,863.06 million by 2028 to expand at 9.5% CAGR during 2023–2028.
Based on country, the North America colorectal cancer diagnostic market is segmented into the U.S., Canada, and Mexico. Our regional analysis states that the US captured 78.6% share of North America colorectal cancer diagnostic market in 2023. It was assessed at US$ 3,392.22 million in 2023 and is likely to hit US$ 5,238.06 million by 2028, exhibiting a CAGR of 12.9% during the forecast period.
North America Colorectal Cancer Diagnostics Market Overview
The North America colorectal cancer diagnostics market is segmented into the US, Canada, and Mexico. According to the American Society of Clinical Oncology, colorectal cancer is the third most common cancer diagnosed in men and women in the US. In 2023, an estimated 153,020 adults in the US will be diagnosed with colorectal cancer. These numbers include 106,970 new cases of colon cancer (54,420 men and 52,550 women) and 46,050 new cases of rectal cancer (27,440 men and 18,610 women). Further, ~1,880,725 people were diagnosed with colorectal cancer in 2020. Although older adults are more prone to colorectal cancer, the incidence of cancer is increasing among younger adults. The lifetime risk of getting affected by colorectal cancer is ~1 in 22 for men and ~1 in 24 for women. The American Society of Clinical Oncology (ASCO) estimated that ~52,550 deaths from colorectal cancer disease will occur in the US in 2023. In 2022, more than 600,000 surgeries were performed annually in the US to treat colon diseases. According to the American Cancer Society, the five-year survival rate for localized colon cancer is 91% and for localized rectal cancer is 90%. Overall, colorectal cancer mortality rates have been declined with better screening and treatment over the past 20 years. Moreover, screening reduces CRC mortality by decreasing incidences of CRC and increasing survival rates. The 2018 American Cancer Society CRC screening guideline recommends that adults aged 45 years and above must undergo regular screening with a high-sensitivity stool-based test or visual examination, depending on patient preference and test availability. In May 2021, the US Preventive Services Task Force changed its colorectal cancer screening recommendation. The age at which adults were at average risk of being diagnosed with colorectal cancer and recommended to begin screening was lowered from 50 to 45. Also, the government of the country supports research on colorectal cancer. The researchers worked to develop an advanced technique that can be used to prevent, detect, and treat colorectal cancer. The Colorectal Cancer Alliance is the world’s largest nonprofit colorectal cancer patient advocacy organization dedicated to funding colorectal cancer research. In 2020, an additional US$ 1.1 million in funding was made to support individual scientists through research grants. Thus, owing to abovementioned factors, the North America colorectal cancer diagnostics market is likely to propel during the forecast period.
North America Colorectal Cancer Diagnostics Strategic Insights
Strategic insights for the North America Colorectal Cancer Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market
North America Colorectal Cancer Diagnostics Report Scope
| Attribute | Details |
| Market Size (2023) | US$ 4,315.86 Million |
| Market Size (2028) | US$ 6,621.26 Million |
| Global CAGR (2023–2028) | 8.9% |
| Historical Data | 2021–2022 |
| Forecast Period | 2024–2028 |
| Segments Covered | By Modality (Imaging Tests, Stool-Based Tests) By End User (Hospitals, Diagnostic Laboratories, Cancer Research Institutes) |
| Regions and Countries Covered | North America (US, Canada, Mexico) |
| Key Companies | Bruker Corp, Clinical Genomics, EDP Biotech, Eiken Chemical, Epigenomics AG, Roche, Illumina, Medtronic, Quest Diagnostics, Siemens Healthineers |
North America Colorectal Cancer Diagnostics Regional Insights
The geographic scope of the North America Colorectal Cancer Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
North America Colorectal Cancer Diagnostics Market Segmentation
The North America colorectal cancer diagnostics market is segmented into modality, end user, and country.
Based on modality, the North America colorectal cancer diagnostics market is bifurcated into imaging tests and stool-based tests. In 2023, the imaging tests segment held a larger share of the North America colorectal cancer diagnostics market. The market for the imaging tests segment is further segmented into colonoscopy, CT colonography, flexible sigmoidoscopy, capsule endoscopy, and others. The market for the stool based tests segment is subsegmented into faecal immunochemical test (fit), guaiac-based faecal occult blood test (gFOBT), and stool DNA test.
Based on end user, the North America colorectal cancer diagnostics market is segmented into hospitals, diagnostic laboratories, cancer research institutes, and others. In 2023, the hospitals segment held the largest share of the North America colorectal cancer diagnostics market.
Based on country, the North America colorectal cancer diagnostics market is segmented into the US, Canada, and Mexico. In 2023, the US accounted for the largest share of the North America colorectal cancer diagnostics market.
Medtronic Plc; Illumina Inc; Clinical Genomics Technologies Pty Ltd; EDP Biotech Corp; Epigenomics AG; F. Hoffmann-La Roche Ltd; Quest Diagnostics Inc; Siemens Healthineers AG; Bruker Corp; and Eiken Chemical Co., Ltd. are among the leading companies operating in the North America colorectal cancer diagnostics market.
Can you see this our reports –
North American Neuromodulation Market – https://postyourarticle.com/north-american-neuromodulation-market-trends-size-segment-and-growth-by-forecast-to-2030-3/
North America Testing, Inspection, and Certification Market – https://sites.google.com/view/businessmarketinsight-61/home
Europe Halal Cosmetics Market – https://businessmarketins02.blogspot.com/2025/04/europe-halal-cosmetics-market-trends_25.html
Europe Events Market – https://www.openpr.com/news/3964271/europe-events-market-trends-size-segment-and-growth
Europe Food Safety Testing Kits Market – https://www.linkedin.com/feed/update/urn:li:groupPost:3225931-7320430279385317376/
About Us:
Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Défense; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications
Author’s Bio
Akshay
Senior Market Research Expert at Business Market Insights