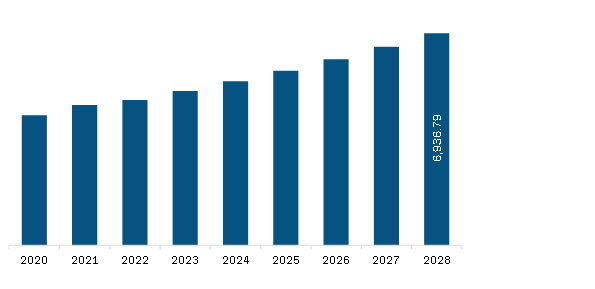
Market Introduction
Point-of-care molecular diagnostics include portable devices, and assays & kits used to detect and diagnose diseases in human samples, such as throat swab, blood, serum, and stool. Molecular diagnostics are shifting from centralized laboratories to decentralized point-of-care molecular testing. Due to its simplicity, convenience, rapid turnaround time, and potential to improve patient outcomes, POCT is rapidly gaining traction.
Moreover, the growing demand for specific viral detection methods that consume less time for timely infection control is expected to bolster the market growth during the forecast period.
📚 𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐒𝐚𝐦𝐩𝐥𝐞 𝐏𝐃𝐅 𝐂𝐨𝐩𝐲@ https://www.businessmarketinsights.com/sample/TIPRE00026190
In Europe, currently, the UK and Russia are the hardest-hit countries by the COVID-19 pandemic, followed by France, Spain, Italy, and Germany. The implementation of drastic measures and travel restrictions, including closing the borders, impacted the import and export of products, especially in the first half of 2020. The continuous spread of the disease has led to an elevated demand for advanced diagnostic solutions in Europe, thus boosting the adoption of point-of-care molecular diagnostic kits. Moreover, uninterrupted investments and business activities by industry players are also boosting the point-of-care molecular diagnostic market growth. In 2020, European Medicines Agency (EMA) approved ~97 medicines for the marketing authorization, of which 39 were novel drugs. In October 2020, Eurofins introduced PCR-based COVID-19 diagnostic tests with at-home, self-sampling options, in the for European market. In January 2020, European Union (EU) commission announced an emergency call, through which it awarded US$ 55.64 million to 18 research projects. Among these, 3 of the projects are receiving a total of US$ 7.39 million to develop effective, rapid point-of-care diagnostics.
📚𝐅𝐮𝐥𝐥 𝐑𝐞𝐩𝐨𝐫𝐭 𝐋𝐢𝐧𝐤 @ https://www.businessmarketinsights.com/reports/europe-point-of-care-molecular-diagnostics-market
𝐓𝐡𝐞 𝐋𝐢𝐬𝐭 𝐨𝐟 𝐂𝐨𝐦𝐩𝐚𝐧𝐢𝐞𝐬
bioMérieux SA
- Hoffmann-La Roche Ltd.
Danaher Corporation
Enzo Biochem, Inc.
Abbott
binx health, Inc.
Meridian BioScience, Inc.
Biocartis
Quidel Corporation
Bio-Rad Laboratories, Inc
- Segments Covered
By Product & Services
- Assays and Kits
- Instruments
- Services and Software
By Technology
- PCR
- Isothermal Nucleic Acid Amplification Technology
- Other Technologies
By Application
- Infectious Diseases
- Oncology
- Hematology
- Prenatal Testing
- Endocrinology
- Other Applications
By End User
- Hospitals and Clinics
- Diagnostic Laboratories
- Research and Academic Institutes
Looking Ahead:
The European POC MDx market is expected to maintain its growth trajectory, driven by:
- Continued focus on pandemic preparedness and response.
- Increasing demand for personalized medicine and decentralized diagnostics.
- Advancements in molecular diagnostic technologies.
- Supportive regulatory frameworks and funding initiatives.
𝐀𝐛𝐨𝐮𝐭 𝐔𝐬:
Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Defense; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications