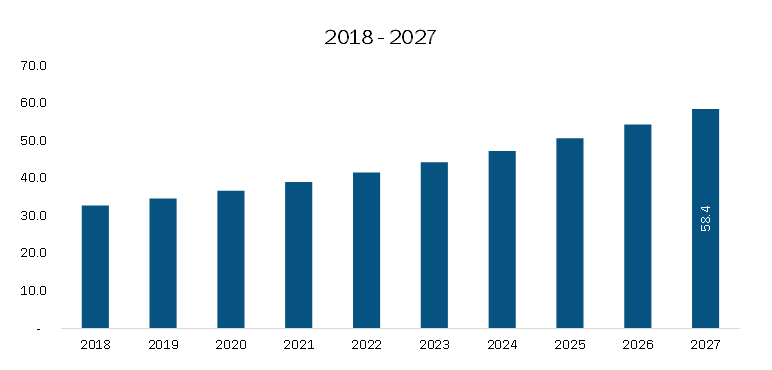
The Europe Clinical Trial Imaging Market is on a promising growth trajectory, with expectations to increase from US$ 501.22 million in 2023 to US$ 705.65 million by 2028, at a compound annual growth rate (CAGR) of 7.1%. This growth is largely driven by the rise in clinical trial activities, especially in the pharmaceutical and biotechnology sectors, where there is an increasing focus on accurately assessing the outcomes of drug development.
𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐏𝐃𝐅 𝐁𝐫𝐨𝐜𝐡𝐮𝐫𝐞 – https://www.businessmarketinsights.com/sample/BMIRE00028612
In-vivo imaging techniques, such as positron emission tomography (PET) and magnetic resonance imaging (MRI), are at the forefront of enhancing the accuracy and efficiency of clinical trials. PET imaging can visualize radio-labeled compounds, allowing for real-time monitoring of targeted drug activities and compound tissue exposure. MRI, on the other hand, provides detailed visualization of human anatomy and can detect drug molecules within the body, enabling researchers to assess their efficacy and toxicity. Other technologies like mass spectrometry imaging (MSI) also contribute by visualizing drug molecules in ex-vivo tissue samples.
As clinical trials become increasingly sophisticated, these imaging modalities help reduce the time and cost associated with obtaining accurate data. They also lead to more successful outcomes in drug development, which is why their use in clinical trial testing is growing rapidly.
Regional Insights:
The market is segmented into several regions within Europe, including the UK, Germany, France, Italy, Spain, and the rest of Europe. Among these, Germany stands out as a key player in the clinical trial landscape. The country benefits from an established infrastructure, a large patient pool, and strong demand for high-quality healthcare, making it an attractive location for clinical research. Moreover, the German government’s funding programs, such as those under the Federal Ministry of Education and Research, are strengthening academic clinical research and fostering the growth of clinical trials.
Germany’s standardized, reliable, and transparent clinical trial approval processes, which feature relatively short study start-up timelines, further contribute to its appeal. In addition, the use of clinical trial imaging software, such as “ERICA” by Pharmtrace, provides a flexible, regulatory-compliant platform for managing medical images in clinical trials, further enhancing the quality and efficiency of the research process.
Key Takeaways:
- Technological Advancements: The use of advanced imaging techniques like PET, MRI, and MSI is pivotal in improving the accuracy and success rates of clinical trials.
- Regional Growth: Countries like Germany, with a strong research infrastructure and regulatory support, play a central role in the European market.
- Strategic Market Positioning: The growing demand for imaging technologies in clinical trials offers opportunities for stakeholders to capitalize on emerging trends, such as regulatory-compliant software platforms and enhanced imaging techniques.
Would you like to dive deeper into any of the technologies mentioned, or explore market opportunities in specific countries like Germany?
Top of Form
About Us:
Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Défense; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications
Author’s Bio:
Akshay
Senior Market Research Expert at Business Market Insights